Enantioselective catalytic β-amination through proton-coupled electron transfer followed by stereocontrolled radical–radical coupling†
Abstract
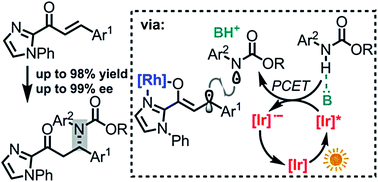
A new mechanistic approach for the catalytic, enantioselective conjugate addition of nitrogen-based nucleophiles to acceptor-substituted alkenes is reported, which is based on a visible light induced and phosphate base promoted transfer of a single electron from a nitrogen nucleophile to a catalyst-bound acceptor-substituted alkene, followed by a stereocontrolled C–N bond formation through stereocontrolled radical–radical coupling. Specifically, N-aryl carbamates are added to the β-position of α,β-unsaturated 2-acyl imidazoles using a visible light activated photoredox mediator in combination with a chiral-at-rhodium Lewis acid catalyst and a weak phosphate base, affording new C–N bonds in a highly enantioselective fashion with enantioselectivities reaching up to 99% ee and >99 : 1 dr for a menthol-derived carbamate. As an application, the straightforward synthesis of a chiral β-amino acid ester derivative is demonstrated.
- This article is part of the themed collection: Celebrating a Century of Excellency in Chemistry at Xiamen University


 Please wait while we load your content...
Please wait while we load your content...