Formation and ligand-based reductive chemistry of bridged bis-alkylidene scandium(iii) complexes†
Abstract
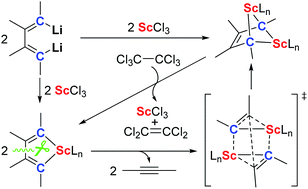
The chemistry of rare-earth carbene and alkylidene complexes including their synthesis, structure and reaction is a challenging issue because of their high reactivity (or instability) and the lack of synthetic methods. In this work, we report the first synthesis of the bridged bis-alkylidene complexes which feature a 2-butene-1,1,4,4-tetraanion and four Sc–C(sp3) bonds by the reaction of 1,4-dilithio-1,3-butadienes with ScCl3. This reaction proceeds via two key intermediates: an isolable scandacyclopentadiene and a proposed scandacyclopropene. The scandacyclopentadiene undergoes β,β′-C–C bond cleavage to generate the scandacyclopropene, which then dimerizes to afford the bridged bis-alkylidene complex via a cooperative double metathesis reaction. Reaction chemistry study of the bridged bis-alkylidene complex reveals their ligand-based reduction reactivity towards different oxidants such as hexachloroethane, disulfide and cyclooctatetraene.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...