Design of peptide-containing N5-unmodified neutral flavins that catalyze aerobic oxygenations†
Abstract
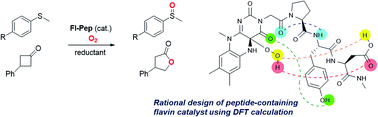
Simulation of the monooxygenation function of flavoenzyme (Fl-Enz) has been long-studied with N5-modified cationic flavins (FlEt+), but never with N5-unmodified neutral flavins (Fl) despite the fact that Fl is genuinely equal to the active center of Fl-Enz. This is because of the greater lability of 4a-hydroperoxy adduct of Fl, FlOOH, compared to those of FlEt+, FlEtOOH, and Fl-Enz, FlOOH-Enz. In this study, Fl incorporated into a short peptide, flavopeptide (Fl-Pep), was designed by a rational top-down approach using a computational method, which could stabilize the corresponding 4a-hydroperoxy adduct (FlOOH-Pep) through intramolecular hydrogen bonds. We report catalytic chemoselective sulfoxidation as well as Baeyer–Villiger oxidation by means of Fl-Pep under light-shielding and aerobic conditions, which are the first Fl-Enz-mimetic aerobic oxygenation reactions catalyzed by Fl under non-enzymatic conditions.


 Please wait while we load your content...
Please wait while we load your content...