MD simulations and QM/MM calculations show that single-site mutations of cytochrome P450BM3 alter the active site’s complexity and the chemoselectivity of oxidation without changing the active species†
Abstract
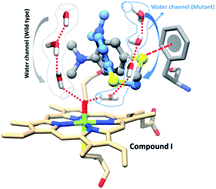
It is a long-standing mechanistic consensus that the mutation of the proton-shuttle mediator Threonine (T) in Cytochrome P450 enzymes severs the water channel and thereby quenches the formation of the active species: the high-valent iron(IV)-oxo porphyrin π-cation radical species, compound I (Cpd I). Using MD simulations and hybrid QM/MM calculations of P450BM3 we demonstrate that this is not the case. Thus, while the original water channel is disrupted in the T268A mutant of the enzyme, a new channel is formed that generates Cpd I. With this new understanding, we address the puzzling regiochemical and kinetic-isotope effect (KIE) results (Volz et al., J. Am. Chem. Soc., 2002, 124, 9724–9725) on the sulfoxidation and N-dealkylation of dimethyl-(4-methylsulfanyl-phenyl)-amine by wild type (WT) P450BM3 and its T268A vs. F87A mutants. We show that the observed variable ratio of S/Me oxidation for these enzymes, vis-à-vis the constant KIE, originates from Cpd I being the sole oxidant. Thus, while the conserved KIE probes the conserved nature of the transition state, the variable regiochemical S/Me ratio reflects the active-site reorganization in the mutants: the shifted location of the new water channel in T268A tightens the binding of the S-end by Cpd I and increases the S/Me ratio, whereas the absence of π-interaction with the S-end in F87A creates a looser binding that lowers the S/Me ratio. Our results match the experimental findings. As such, this study sheds light on puzzling experimental results, and may shift a central paradigm in P450 research. The broader implication on enzymatic research is that a single-site mutation is not a localised alteration but one that may lead to a profound change in the active site, sufficiently so as to change the chemoselectivity of catalyzed reactions.


 Please wait while we load your content...
Please wait while we load your content...