A simple and traceless solid phase method simplifies the assembly of large peptides and the access to challenging proteins†
Abstract
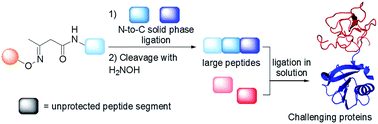
Chemical protein synthesis gives access to well-defined native or modified proteins that are useful for studying protein structure and function. The majority of proteins synthesized up to now have been produced using native chemical ligation (NCL) in solution. Although there are significant advantages to assembling large peptides or proteins by solid phase ligation, reports of such approaches are rare. We report a novel solid phase method for protein synthesis which relies on the chemistry of the acetoacetyl group and ketoxime ligation for the attachment of the peptide to the solid support, and on a tandem transoximation/rearrangement process for the detachment of the target protein. Importantly, we show that the combination of solid phase and solution ligation techniques facilitates the production of a challenging and biologically active protein made of 180 amino acids. We show also that the solid phase method enables the purification of complex peptide segments through a chemoselective solid phase capture/release approach.


 Please wait while we load your content...
Please wait while we load your content...