Catalyst-controlled polycondensation of glycerol with diacyl chlorides: linear polyesters from a trifunctional monomer†
Abstract
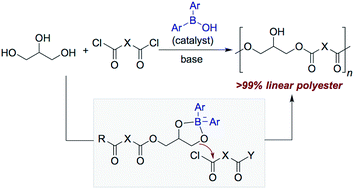
Diarylborinic acids catalyze the formation of linear polyesters from glycerol, a trifunctional, carbohydrate-based monomer. The selective activation of 1,2-diols over isolated alcohols by the organoboron catalyst results in polymers that are essentially free of branching or cross-linking and possess a high fraction of 1,3-enchained glycerol units, as assessed by 1H and 13C NMR spectroscopy. The ability to generate well-defined polyester architectures from glycerol is significant in light of the numerous applications of such macromolecules, particularly in the biomedical area. Isomerization, post-polymerization functionalization and controlled cross-linking reactions of the obtained linear poly(glycerol esters) are demonstrated.


 Please wait while we load your content...
Please wait while we load your content...