Efficient syntheses of (−)-crinine and (−)-aspidospermidine, and the formal synthesis of (−)-minfiensine by enantioselective intramolecular dearomative cyclization†
Abstract
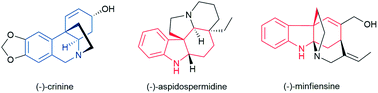
Polycyclic alkaloids bearing all-carbon quaternary centers possess a diversity of biological activities and are challenging targets in natural product synthesis. The development of a general and asymmetric catalytic method applicable to the efficient syntheses of a series of complex polycyclic alkaloids remains highly desirable in synthetic chemistry. Herein we describe an efficient palladium-catalyzed enantioselective dearomative cyclization which is capable of synthesizing two important classes of tricyclic nitrogen-containing skeleton, chiral dihydrophenanthridinone and dihydrocarbazolone derivatives bearing all-carbon quaternary centers, in excellent yields and enantioselectivities. The P-chiral monophosphorus ligand AntPhos is crucial for the reactivity and enantioselectivity, and the choice of the N-phosphoramide protecting group is essential for the desired chemoselectivity. This method has enabled the enantioselective total syntheses of three distinctive and challenging biologically important polycyclic alkaloids, specifically a concise and gram-scale synthesis of (−)-crinine, an efficient synthesis of indole alkaloid (−)-aspidospermidine and a formal enantioselective synthesis of (−)-minfiensine.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...