Hydrogen generation from methanol at near-room temperature†
Abstract
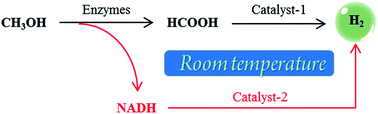
As a promising hydrogen storage medium methanol has many advantages such as a high hydrogen content (12.5 wt%) and low-cost. However, conventional methanol–water reforming methods usually require a high temperature (>200 °C). In this research, we successfully designed an effective strategy to fully convert methanol to hydrogen for at least 1900 min (∼32 h) at near-room temperature. The strategy involves two main procedures, which are CH3OH → HCOOH → H2 and CH3OH → NADH → H2. HCOOH and the reduced form of nicotinamide adenine dinucleotide (NADH) are simultaneously produced through the dehydrogenation of methanol by the cooperation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Subsequently, HCOOH is converted to H2 by a new iridium polymer complex catalyst and an enzyme mimic is used to convert NADH to H2 and nicotinamide adenine dinucleotide (NAD+). NAD+ can then be reconverted to NADH by repeating the dehydrogenation of methanol. This strategy and the catalysts invented in this research can also be applied to hydrogen production from other small organic molecules (e.g. ethanol) or biomass (e.g. glucose), and thus will have a high impact on hydrogen storage and applications.


 Please wait while we load your content...
Please wait while we load your content...