Perhydrohelicenes and other diamond-lattice based hydrocarbons: the choreography of inversion†
Abstract
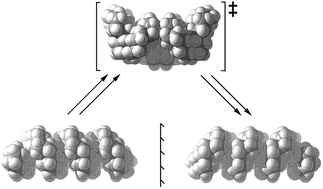
Overall inversion in fused cyclohexane oligomers 2, 3, and 4 (all based on cis-decalin 1) occurs by a rolling process involving no more than two adjacent rings in twist-boat conformations at any time. These inverting rings move along the oligomer in processes that are precisely choreographed by the adjacent chairs. Actual inversion mechanisms can be stepwise [CC → TC → TT → C′T → C′C′], as for cis-decalin, but it is shown that a concerted alternative [CC → TC → C′T → C′C′] is enforced in 2. The all-cis,anti,cis-isomers of perhydrohelicenes 4 are based on the diamond lattice and have remarkably low strain energies. Helix inversion in 4 is compared with that in helicenes 5. For both, the intermediates and transition states have shapes broadly like kinked old-style telephone cables. In both cases barriers increase with the length of the system to eventually reach a plateau value of ca. 120 kJ mol−1 for 4, much lower than that for 5 (320–350 kJ mol−1). While rolling inversion only requires two adjacent rings in twist-boat conformations at any instant, inversion in propellane 6 requires all three rings be converted to twist-boats, and the S4 symmetric hydrocarbon 7 requires all four rings to be converted to twist-boats. As a consequence, 7 probably has the highest barrier of any non-oligomeric cis-decalin derived structure (87.3 kJ mol−1 at B3LYP/6-31G*).


 Please wait while we load your content...
Please wait while we load your content...