Pivalophenone imine as a benzonitrile surrogate for directed C–H bond functionalization†
Abstract
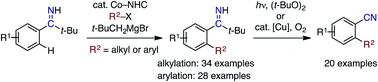
Pivalophenone N–H imine has been found to serve as a prominent substrate for directed C–H alkylation and arylation reactions with alkyl bromides and aryl chlorides, respectively, under cobalt–N-heterocyclic carbene (NHC) catalysis. Unlike the case of the parent pivalophenone imine, the increased steric bulk of the resulting ortho-substituted pivalophenone imines allows them to undergo clean imine-to-nitrile conversion under peroxide photolysis or aerobic copper catalysis conditions. Overall, these two-step transformations offer convenient synthetic methods for ortho-functionalized benzonitriles.


 Please wait while we load your content...
Please wait while we load your content...