A new route to N-aromatic heterocycles from the hydrogenation of diesters in the presence of anilines†
Abstract
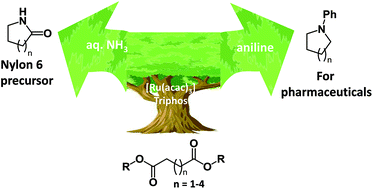
The hydrogenation of dicarboxylic acids and their esters in the presence of anilines provides a new synthesis of heterocycles. [Ru(acac)3] and 1,1,1-tris(diphenylphosphinomethyl)ethane (triphos) gave good to excellent yields of the cyclic amines at 220 °C. When aqueous ammonia was used with dimethyl 1,6-hexadienoic acid, ε-caprolactam was obtained in good yield. A side reaction involving alkylation of the amine by methanol was suppressed by using diesters derived from longer chain and branched alcohols. Hydrogenation of optically pure diesters (dimethyl (R)-2-methylbutanedioate and dimethyl (S)-2-methylbutanedioate) with aniline afforded racemic 3-methyl-1-phenylpyrrolidine in 78% yield.
- This article is part of the themed collection: ISACS: Challenges in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...