Cyclohexa-1,4-dienes in transition-metal-free ionic transfer processes
Abstract
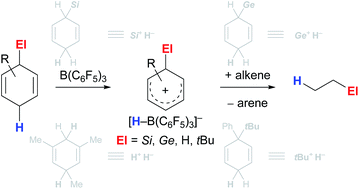
Safe- and convenient-to-handle surrogates of hazardous chemicals are always in demand. Recently introduced cyclohexa-1,4-dienes with adequate substitution fulfil this role as El+/H− equivalents in B(C6F5)3-catalysed transfer reactions of El–H to π- and σ-donors (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). Surrogates of Si–H/Ge–H, H–H and even C–H bonds have been designed and successfully applied to ionic transfer hydrosilylation/hydrogermylation, hydrogenation and hydro-tert-butylation, respectively. These processes and their basic principles are summarised in this Minireview. The similarities and differences between these transfer reactions as well as the challenges associated with these transformations are discussed.
N). Surrogates of Si–H/Ge–H, H–H and even C–H bonds have been designed and successfully applied to ionic transfer hydrosilylation/hydrogermylation, hydrogenation and hydro-tert-butylation, respectively. These processes and their basic principles are summarised in this Minireview. The similarities and differences between these transfer reactions as well as the challenges associated with these transformations are discussed.


 Please wait while we load your content...
Please wait while we load your content...