Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs†
Abstract
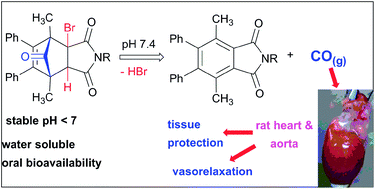
A prodrug strategy for the release of the gasotransmitter CO at physiological pH, based upon 3a-bromo-norborn-2-en-7-one Diels–Alder cycloadducts of 2-bromomaleimides and 2,5-dimethyl-3,4-diphenylcyclopentadienone has been developed. Examples possessing protonated amine and diamine groups showed good water solubility and thermal stability. Half-lives for CO-release in TRIS-sucrose buffer at pH 7.4 ranged from 19 to 75 min at 37 °C and 31 to 32 h at 4 °C. Bioavailability in rats was demonstrated by oral gavage and oCOm-21 showed a dose dependent vasorelaxant effect in pre-contracted rat aortic rings with an EC50 of 1.6 ± 0.9 μM. Increased intracellular CO levels following oCOm-21 exposure were confirmed using a CO specific fluorescent probe.


 Please wait while we load your content...
Please wait while we load your content...