Recent developments in the use of aza-Heck cyclizations for the synthesis of chiral N-heterocycles
Abstract
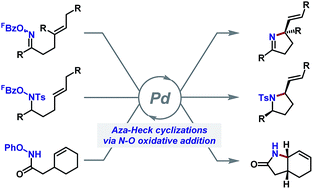
Aza-Heck cyclizations are an emerging method for the construction of chiral N-heterocyclic systems. In these processes, an activated N–O bond replaces the C–X bond (X = halide, OTf) used in conventional Heck reactions, with the associated aza-Pd(II)-intermediate engaging pendant alkenes in a Heck-like manner. This perspective article commences with an historical overview of the area, which stems from Narasaka's seminal studies using oxime esters as the initiating motif. The scope and mechanism of associated chiral N-heterocyclic methodologies are then outlined, including cascade processes that enable diverse alkene 1,2-carboaminations. The recent emergence of new N–O donors and the realization of highly enantioselective aza-Heck cyclizations are then discussed. Collectively, these studies suggest that the aza-Heck approach can underpin a broad family of redox-neutral and enantioselective C–N bond forming processes.


 Please wait while we load your content...
Please wait while we load your content...