Metal exchange in lithiocuprates: implications for our understanding of structure and reactivity†
Abstract
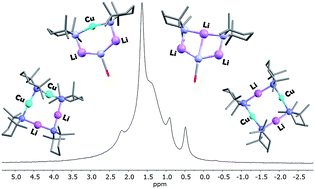
New reagents have been sought for directed ortho cupration in which the use of cyanide reagents is eliminated. CuOCN reacts with excess TMPLi (TMP = 2,2,6,6-tetramethylpiperidide) in the presence of limited donor solvent to give crystals that are best represented as (TMP)2Cu0.1Li0.9(OCN)Li2(THF) 8, whereby both Lipshutz-type lithiocuprate (TMP)2Cu(OCN)Li2(THF) 8a and trinuclear (TMP)2(OCN)Li3(THF) 8b are expressed. Treatment of a hydrocarbon solution of TMP2CuLi 9a with LiOCN and THF gives pure 8a. Meanwhile, formation of 8b is systematized by reacting (TMPH2)OCN 10 with TMPH and nBuLi to give (TMP)2(OCN)Li3(THF)211. Important to the attribution of lower/higher order bonding in lithiocuprate chemistry is the observation that in crystalline 8, amide-bridging Cu and Li demonstrate clear preferences for di- and tricoordination, respectively. A large excess of Lewis base gives an 8-membered metallacycle that retains metal disorder and analyses as (TMP)2Cu1.35Li0.659 in the solid state. NMR spectroscopy identifies 9 as a mixture of (TMP)2CuLi 9a and other copper-rich species. Crystals from which the structure of 8 was obtained dissolve to yield evidence for 8b coexisting in solution with in situ-generated 9a, 11 and a kinetic variant on 9a (i-9a), that is best viewed as an agglomerate of TMPLi and TMPCu. Moving to the use of DALi (DA = diisopropylamide), (DA)2Cu0.09Li0.91(Br)Li2(TMEDA)212 (TMEDA = N,N,N′,N′-tetremethylethylenediamine) is isolated, wherein (DA)2Cu(Br)Li2(TMEDA)212a exhibits lower-order Cu coordination. The preparation of (DA)2Li(Br)Li2(TMEDA)212b was systematized using (DAH2)Br, DAH and nBuLi. Lastly, metal disorder is avoided in the 2 : 1 lithium amide : Lipshutz-type monomer adduct (DA)4Cu(OCN)Li4(TMEDA)213.


 Please wait while we load your content...
Please wait while we load your content...