Kinase-templated abiotic reaction†
Abstract
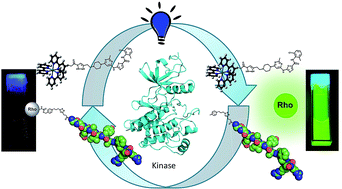
Protein kinases are quintessential regulators of cellular function. Numerous pathologies are intimately linked to the dysregulated activity of a particular protein kinase. Herein we report a technology based on a proximity-induced chemical transformation that enables the detection and imaging of specific kinases. Using two probes that target the nucleotide-binding site and substrate binding site of a target kinase respectively, the reagents appended on the probes are brought within reactive distance thereby enabling the chemical transformation. The reaction used for sensing is a ruthenium-photocatalyzed reduction of a pyridinium immolative linker, which uncages a fluorophore (rhodamine). We demonstrate that this technology can be used to discriminate between closely related kinases with a high signal to noise ratio. We further demonstrate that the technology operates within the complexity of a cellular context with a good correlation between the level of kinase activity and fluorescence output.


 Please wait while we load your content...
Please wait while we load your content...