Double quick, double click reversible peptide “stapling”†
Abstract
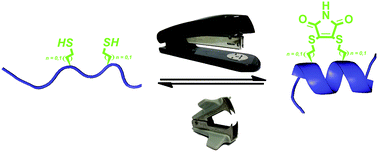
The development of constrained peptides for inhibition of protein–protein interactions is an emerging strategy in chemical biology and drug discovery. This manuscript introduces a versatile, rapid and reversible approach to constrain peptides in a bioactive helical conformation using BID and RNase S peptides as models. Dibromomaleimide is used to constrain BID and RNase S peptide sequence variants bearing cysteine (Cys) or homocysteine (hCys) amino acids spaced at i and i + 4 positions by double substitution. The constraint can be readily removed by displacement of the maleimide using excess thiol. This new constraining methodology results in enhanced α-helical conformation (BID and RNase S peptide) as demonstrated by circular dichroism and molecular dynamics simulations, resistance to proteolysis (BID) as demonstrated by trypsin proteolysis experiments and retained or enhanced potency of inhibition for Bcl-2 family protein–protein interactions (BID), or greater capability to restore the hydrolytic activity of the RNAse S protein (RNase S peptide). Finally, use of a dibromomaleimide functionalized with an alkyne permits further divergent functionalization through alkyne–azide cycloaddition chemistry on the constrained peptide with fluorescein, oligoethylene glycol or biotin groups to facilitate biophysical and cellular analyses. Hence this methodology may extend the scope and accessibility of peptide stapling.
- This article is part of the themed collection: Most downloaded articles of 2017: Analytical, Biological and Medicinal Chemistry


 Please wait while we load your content...
Please wait while we load your content...