Enantioselective total synthesis of (−)-colchicine, (+)-demecolcinone and metacolchicine: determination of the absolute configurations of the latter two alkaloids†
Abstract
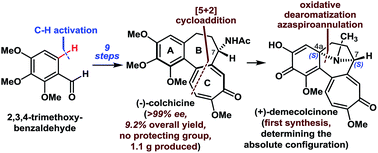
Here, we describe a concise, enantioselective, and scalable synthesis of (−)-colchicine (9.2% overall yield, >99% ee). Moreover, we have also achieved the first syntheses of (+)-demecolcinone and metacolchicine, and determined their absolute configurations. The challenging tricyclic 6-7-7 core of colchicinoids was efficiently introduced using an intramolecular oxidopyrylium-mediated [5 + 2] cycloaddition reaction. Notably, the synthesized colchicinoid 23 exhibited potent inhibitory activity toward the cell growth of human cancer cell lines (IC50 = ∼3.0 nM), and greater inhibitory activity towards microtubule assembly than colchicine, making it a promising lead in the search for novel anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...