Catechol adsorption on graphene nanoplatelets: isotherm, flat to vertical phase transition and desorption kinetics†
Abstract
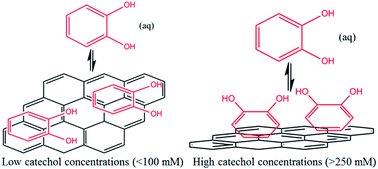
The adsorption of catechol (1,2-dihydroxybenzene) on graphene nanoplatelets (GNPs) is investigated electrochemically and spectroscopically. The reversible adsorption of catechol on GNPs is Langmuirian with an adsorption constant of (0.2 ± 0.002) mM−1 at low adsorbate concentrations (≤100 mM). At higher concentrations (>100 mM) the adsorption of catechol on GNPs is shown to undergo a flat to vertical concentration driven phase transition. The kinetics of desorption are measured with a single particle electrochemical technique. The study of individual impacts allows the determination of the rate of catechol desorption from GNPs to be k = 0.08 ± 0.01 s−1 with first order kinetics. The method provides a powerful and efficient generic approach to study adsorption and, importantly, desorption of molecules on nanomaterials, as well as giving insight into the modification process.


 Please wait while we load your content...
Please wait while we load your content...