Identification of new quorum sensing autoinducer binding partners in Pseudomonas aeruginosa using photoaffinity probes†
Abstract
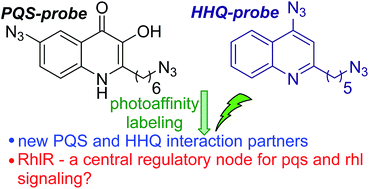
Many bacterial species, including the human pathogen Pseudomonas aeruginosa, employ a mechanism of intercellular communication known as quorum sensing (QS), which is mediated by signalling molecules termed autoinducers. The Pseudomonas Quinolone Signal (PQS) and 2-Heptyl-3H-4-Quinolone (HHQ) are autoinducers in P. aeruginosa, and they are considered important factors in the progress of infections by this clinically relevant organism. Herein, we report the development of HHQ and PQS photoaffinity-based probes for chemical proteomic studies. Application of these probes led to the identification of previously unsuspected putative HHQ and PQS binders, thereby providing new insights into QS at a proteomic level and revealing potential new small molecule targets for virulence attenuation strategies. Notably, we found evidence that PQS binds RhlR, the cognate receptor in the Rhl QS sub-system of P. aeruginosa. This is the first indication of interaction between the Rhl and PQS systems at the protein/ligand level, which suggests that RhlR should be considered a highly attractive target for antivirulence strategies.


 Please wait while we load your content...
Please wait while we load your content...