Enantioselective palladium-catalyzed diboration of 1,1-disubstituted allenes†
Abstract
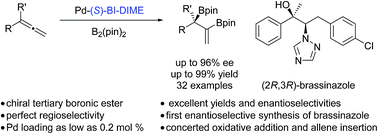
A practical and enantioselective palladium-catalyzed diboration of 1,1-disubstituted allenes is developed by employing a P-chiral monophosphorus ligand, BI-DIME. A series of diboronic esters containing a chiral tertiary boronic ester moiety are formed in excellent yields and ee’s with the palladium loading as low as 0.2 mol%. DFT calculations revealed a concerted mechanism of oxidative addition of bis(pinacolato)diboron and allene insertion, as well as a critical dispersion effect on the origins of the enantioselectivity. The method is successfully applied to the concise and enantioselective synthesis of brassinazole.


 Please wait while we load your content...
Please wait while we load your content...