Cu/Mn bimetallic catalysis enables carbonylative Suzuki–Miyaura coupling with unactivated alkyl electrophiles†
Abstract
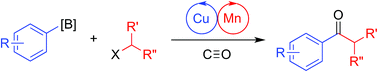
A bimetallic system consisting of Cu-carbene and Mn-carbonyl co-catalysts was employed for carbonylative C–C coupling of arylboronic esters with alkyl halides, allowing for the convergent synthesis of ketones. The system operates under mild conditions and exhibits complementary reactivity to Pd catalysis. The method is compatible with a wide range of arylboronic ester nucleophiles and proceeds smoothly for both primary and secondary alkyl iodide electrophiles. Preliminary mechanistic experiments corroborate a hypothetical catalytic mechanism consisting of co-dependent cycles wherein the Cu-carbene co-catalyst engages in transmetallation to generate an organocopper nucleophile, while the Mn-carbonyl co-catalyst activates the alkyl halide electrophile by single-electron transfer and then undergoes reversible carbonylation to generate an acylmanganese electrophile. The two cycles then intersect with a heterobimetallic, product-releasing C–C coupling step.


 Please wait while we load your content...
Please wait while we load your content...