Versatile telluracycle synthesis via the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds†
Abstract
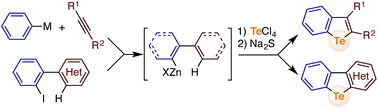
We report herein a new approach for the synthesis of tellurium-bridged aromatic compounds based on the sequential electrophilic telluration of C(sp2)–Zn and C(sp2)–H bonds with tellurium(IV) chlorides. A combination of transition metal-catalyzed (migratory) arylmetalation of alkynes and sequential telluration allows for the expedient construction of a library of functionalized benzo[b]tellurophenes. Furthermore, a variety of heteroarene-fused benzotellurophenes and other novel tellurium-embedded polycyclic aromatics can be readily synthesized from the corresponding 2-iodoheterobiaryls.


 Please wait while we load your content...
Please wait while we load your content...