A complex stereochemical relay approach to the antimalarial alkaloid ocimicide A1. Evidence for a structural revision†
Abstract
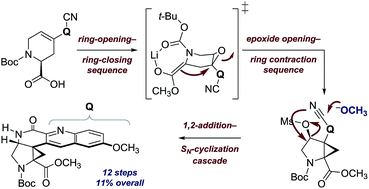
Ocimicide A1 (1) and the semisynthetic derivative ocimicide A2 (2) are highly potent antimalarial agents efficacious against chloroquine-sensitive and -resistant Plasmodium falciparum strains with IC50 values in the nanomolar and picomolar range, respectively. Members of this family have demonstrated radical cure in rhesus monkeys, without detectable toxicity, but their structure–function relationships and mechanism of action are unknown. Herein we describe a twelve-step synthesis of an advanced N-acylated pentacyclic precursor to the proposed structure of 1 (11% overall yield). Instability and poor P. falciparum growth inhibition of the corresponding free donor–acceptor cyclopropylamine, and large discrepancies between reported and both experimental and DFT-calculated 13C chemical shifts and coupling constants, suggest that substantial revision of the proposed structures may be necessary.
- This article is part of the themed collection: ISACS19: Challenges in Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...