CuH-catalysed hydroamination of arylalkynes with hydroxylamine esters – a computational scrutiny of rival mechanistic pathways†
Abstract
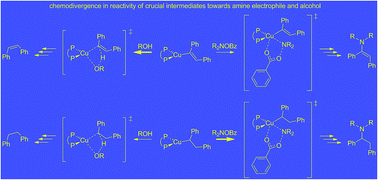
An in-depth computational mechanistic probe of the CuH-mediated hydroamination of internal arylalkynes with an archetype hydroxylamine ester and hydrosilane by a (Xantphos)CuH catalyst (Xantphos ≡ {P^P} ≡ 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene) is presented. This first comprehensive computational study of the CuH-mediated electrophilic alkyne hydroamination has identified the most accessible pathway for the rival avenues for direct and reductive hydroamination. The mechanistic picture derived from smooth energy profiles obtained by employing a reliable computational protocol applied to a realistic catalyst model conforms to all available experimental data. The crucial vinyl- and alkylcopper intermediates were found to display a distinct chemodivergence in their reactivity towards amine electrophile and alcohol, which ensures the successful formation of α-branched alkylamines together with (E)-enamines. On the one hand, the vinylcopper is somewhat preferably approached by the alcohol, thereby rendering the reductive hydroamination avenue favourable in the presence of both amine electrophile and alcohol. In contrast, the greater kinetic demands for protonation versus electrophilic amination predicted for the alkylcopper prevents the reductive hydroamination avenue to become non-productive. Electronically modified hydroxylamine esters are found to influence the chemoselectivity in reactivity towards amine electrophile and alcohol achievable for the vinyl- and alkylcopper, thereby offering an opportunity for process improvement.

 Please wait while we load your content...
Please wait while we load your content...