Enantioselective γ-borylation of unsaturated amides and stereoretentive Suzuki–Miyaura cross-coupling†
Abstract
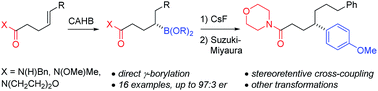
The rhodium-catalyzed, directed catalytic asymmetric hydroboration of γ,δ-unsaturated amides affords a direct route to chiral acyclic secondary γ-borylated carbonyl derivatives in high enantiomeric purity. In contrast to a similar β-borylated amide derivative, the γ-borylated amide undergoes Suzuki–Miyaura cross-coupling with stereoretention. The utility of the boronic ester products is further illustrated by other stereospecific C–B bond transformations leading to γ-amino acid derivatives, 1,4-amino alcohols, and 5-substituted-γ-lactone and γ-lactam ring systems.


 Please wait while we load your content...
Please wait while we load your content...