Characterizing the structural ensemble of γ-secretase using a multiscale molecular dynamics approach†
Abstract
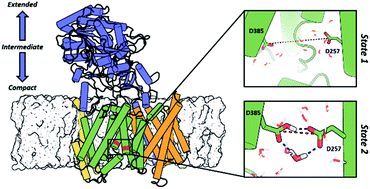
γ-Secretase is an intramembrane-cleaving aspartyl protease that plays an essential role in the processing of a variety of integral membrane proteins. Its role in the ultimate cleavage step in the processing of amyloid precursor protein to form amyloid-β (Aβ) peptide makes it an important therapeutic target in Alzheimer's disease research. Significant recent advances have been made in structural studies of this critical membrane protein complex. However, details of the mechanism of activation of the enzyme complex remain unclear. Using a multiscale computational modeling approach, combining multiple coarse-grained microsecond dynamic trajectories with all-atom models, the structure and two conformational states of the γ-secretase complex were evaluated. The transition between enzymatic state 1 and state 2 is shown to critically depend on the protonation states of the key catalytic residues Asp257 and Asp385 in the active site domain. The active site formation, related to our γ-secretase state 2, is observed to involve a concerted movement of four transmembrane helices from the catalytic subunit, resulting in the required localization of the catalytic residues. Global analysis of the structural ensemble of the enzyme complex was used to identify collective fluctuations important to the mechanism of substrate recognition and demonstrate that the corresponding fluctuations observed were uncorrelated with structural changes associated with enzyme activation. Overall, this computational study provides essential insight into the role of structure and dynamics in the activation and function of γ-secretase.


 Please wait while we load your content...
Please wait while we load your content...