Manganese complex-catalyzed oxidation and oxidative kinetic resolution of secondary alcohols by hydrogen peroxide†
Abstract
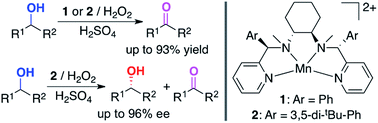
The highly efficient catalytic oxidation and oxidative kinetic resolution (OKR) of secondary alcohols has been achieved using a synthetic manganese catalyst with low loading and hydrogen peroxide as an environmentally benign oxidant in the presence of a small amount of sulfuric acid as an additive. The product yields were high (up to 93%) for alcohol oxidation and the enantioselectivity was excellent (>90% ee) for the OKR of secondary alcohols. Mechanistic studies revealed that alcohol oxidation occurs via hydrogen atom (H-atom) abstraction from an α-CH bond of the alcohol substrate and a two-electron process by an electrophilic Mn–oxo species. Density functional theory calculations revealed the difference in reaction energy barriers for H-atom abstraction from the α-CH bonds of R- and S-enantiomers by a chiral high-valent manganese–oxo complex, supporting the experimental result from the OKR of secondary alcohols.


 Please wait while we load your content...
Please wait while we load your content...