Chemical synthesis of a homoserine-mutant of the antibacterial, head-to-tail cyclized protein AS-48 by α-ketoacid–hydroxylamine (KAHA) ligation†
Abstract
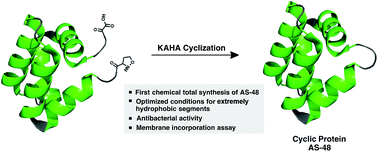
An antibacterial cyclic AS-48 protein was chemically synthesized by α-ketoacid–hydroxylamine (KAHA) ligation. Initial challenges associated with the exceptionally hydrophobic segments arising from the amphiphilic nature of the protein were resolved by the development of bespoke reaction conditions for hydrophobic segments, using hexafluoroisopropanol (HFIP) as a co-solvent. The synthetic protein displays similar biological activity and properties to those of the native protein. To support the current understanding of its antibacterial mode of action, we demonstrate the ability of AS-48 to be incorporated into synthetic multilamellar vesicles (MLVs).


 Please wait while we load your content...
Please wait while we load your content...