Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides†
Abstract
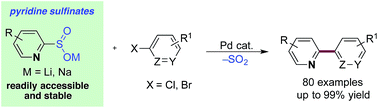
Pyridine rings are ubiquitous in drug molecules; however, the pre-eminent reaction used to form carbon–carbon bonds in the pharmaceutical industry, the Suzuki–Miyaura cross-coupling reaction, often fails when applied to these structures. This phenomenon is most pronounced in 2-substituted pyridines, and results from the difficulty in preparing, the poor stability of, and low efficiency in reactions of pyridine-2-boronates. We demonstrate that by replacing these boronates with pyridine-2-sulfinates, a cross-coupling process of unrivalled scope and utility is realized. The corresponding 3- and 4-substituted pyridine variants are also efficient coupling partners. In addition, we apply these sulfinates in a library format to the preparation of medicinally relevant derivatives of the drugs varenicline (Chantix) and mepyramine (Anthisan).
- This article is part of the themed collection: Most downloaded articles of 2017: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...