Macrocycle-assisted synthesis of non-stoichiometric silver(i) halide electrocatalysts for efficient chlorine evolution reaction†
Abstract
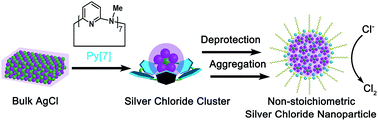
The electrocatalytic oxidation of chloride to chlorine is a fundamental and important electrochemical reaction in industry. Herein we report the synthesis of non-stoichiometric silver halide nanoparticles through a novel macrocycle-assisted bulk-to-cluster-to-nano transformation. The acquired positively charged nanoparticles expedite chloride transportation by electrostatic attraction and facilitate the formation of silver polychloride catalytic species on the surface, thus functioning as efficient and selective electrocatalysts for the chlorine evolution reaction (CER) at a very low overpotential and within a wide concentration range of chloride. The formation of uncommon non-stoichiometric nanoparticles prevents the formation of a AgCl precipitate and exposes more coordination unsaturated silver atoms to catalyze CER, finally causing a large enhancement of the atomic catalytic efficiency of silver. This study showcases a promising approach to achieve efficient catalysts from a bottom-up design.


 Please wait while we load your content...
Please wait while we load your content...