Anion–π catalysis: bicyclic products with four contiguous stereogenic centers from otherwise elusive diastereospecific domino reactions on π-acidic surfaces†
Abstract
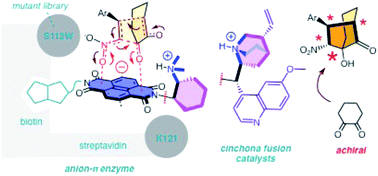
Anion–π interactions have been introduced recently to catalysis. The idea of stabilizing anionic intermediates and transition states on π-acidic surfaces is a new fundamental concept. By now, examples exist for asymmetric enolate, enamine, iminium and transamination chemistry, and the first anion–π enzyme has been created. Delocalized over large aromatic planes, anion–π interactions appear particularly attractive to stabilize extensive long-distance charge displacements during domino processes. Moving on from the formation of cyclohexane rings with five stereogenic centers in one step on a π-acidic surface, we here focus on asymmetric anion–π catalysis of domino reactions that afford bicyclic products with quaternary stereogenic centers. Catalyst screening includes a newly synthesized, better performing anion–π version of classical organocatalysts from cinchona alkaloids, and anion–π enzymes. We find stereoselectivities that are clearly better than the best ones reported with conventional catalysts, culminating in unprecedented diastereospecificity. Moreover, we describe achiral salts as supramolecular chirality enhancers and report the first artificial enzyme that operates in neutral water with anion–π interactions, i.e., interactions that are essentially new to enzymes. Evidence in support of contributions of anion–π interactions to asymmetric catalysis include increasing diastereo- and enantioselectivity with increasing rates, i.e., asymmetric transition-state stabilization in the presence of π-acidic surfaces and inhibition with the anion selectivity sequence NO3− > Br− > BF4− > PF6−.
- This article is part of the themed collection: ISACS18: Challenges in Organic Materials and Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...