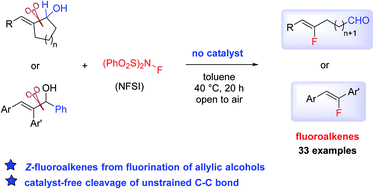
Divergent reactivities in fluoronation of allylic alcohols: synthesis of Z-fluoroalkenes via carbon–carbon bond cleavage†
Abstract
An unconventional cleavage of an unstrained carbon–carbon bond in allylic alcohols can be induced by the use of N-fluorobenzenesulfonimide (NFSI) under catalyst-free conditions. By using this simple procedure, a wide range of functionalized Z-fluoroalkenes can be accessed in high yield and selectivity from cyclic and acyclic allylic alcohols.

 Please wait while we load your content...
Please wait while we load your content...