Denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids†
Abstract
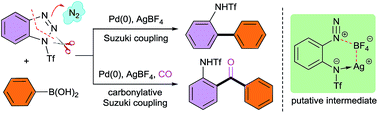
Unprecedented palladium-catalyzed denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids have been realized, which afforded structurally diverse ortho-amino-substituted biaryl and biaryl ketone derivatives. The key to this success is due to the development of a rationally designed strategy to achieve the ring opening of benzotriazoles with a synergistic activating–stabilizing effect, which enables the in situ generation of the corresponding ortho-amino-arenediazonium species. The present work opens up a new avenue to utilize benzotriazoles as synthetic equivalents of ortho-amino-arenediazoniums, which otherwise could not be directly accessed by existing synthetic methods.


 Please wait while we load your content...
Please wait while we load your content...