Catalytic asymmetric synthesis of CF3-substituted tertiary propargylic alcohols via direct aldol reaction of α-N3 amide†
Abstract
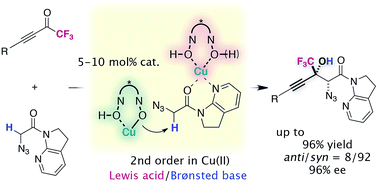
Organofluorine compounds are found in several important classes of chemicals, such as pharmaceuticals, agrochemicals, and functional materials. Chemists have been immensely interested in the development of methodologies for expeditious access to fluorine containing building blocks. In this study, we report a new method for the catalytic asymmetric synthesis of CF3-substituted tertiary propargylic alcohols with two contiguous stereogenic centers via the direct aldol reaction of an α-N3 amide to trifluoromethyl ketones. The key to the success of this method is the identification of a catalyst comprising Cu(II)/chiral hydroxamic acid to promote the desired aldol reaction, constructing a tetrasubstituted carbon in a highly stereoselective fashion. Despite substantial prior advances in asymmetric catalysis, this class of catalysts has not been utilized for the formation of carbon–carbon bond-forming reactions. Our mechanistic study sheds light on the unique profile of this catalytic system, where the Cu(II) complex plays a bifunctional role of serving as a Lewis acid and a Brønsted base. Furthermore, the densely functionalized aldol adducts undergo chemoselective transformations, affording a series of fluorine containing chiral building blocks with widespread application.
- This article is part of the themed collection: Most downloaded articles of 2017: Energy and Catalysis


 Please wait while we load your content...
Please wait while we load your content...