A DFT calculation-inspired Rh(i)-catalyzed reaction via suppression of α-H shift in α-alkyldiazoacetates†
Abstract
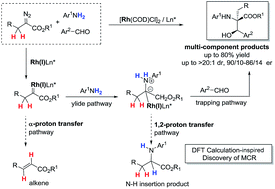
Metal-associated carbenes from diazo compounds promote many useful chemistry transformations in modern organic chemistry. However, compared to α-aryldiazoacetate-derived carbenes (ArDCs), the synthetic application of α-alkyldiazoacetate-derived carbenes (AlDCs) is greatly limited due to intramolecular α-H transfer (elimination) that results in alkenes as the main by-products. An intriguing α-alkyldiazoacetate-involved three-component reaction has been developed following DFT calculation inspiration to provide β-hydroxyl α-alkyl-α-amino acid derivatives in good yields. The intramolecular α-H shift of an α-alkyldiazoacetate-derived carbene was successfully suppressed by the association of a Rh(I) complex to form the corresponding active ammonium ylide, which was trapped before the fast 1,2-H transfer process. A Rh(I)-chiral diene complex was identified as an effective catalyst to give an asymmetric version of the reaction with good enantioselectivity. This reaction provides insight into extending the efficient transformation of α-alkyldiazoacetate-derived carbenes and their synthetic application.


 Please wait while we load your content...
Please wait while we load your content...