Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery†
Abstract
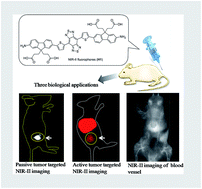
Though high brightness and biocompatible small NIR-II dyes are highly desirable in clinical or translational cancer research, their fluorescent cores are relatively limited and their synthetic processes are somewhat complicated. Herein, we have explored the design and synthesis of novel NIR-II fluorescent materials (H1) without tedious chromatographic isolation with improved fluorescence performance (QY ≈ 2%) by introducing 2-amino 9,9-dialkyl-substituted fluorene as a donor into the backbone. Several types of water-soluble and biocompatible NIR-II probes: SXH, SDH, and H1 NPs were constructed via different chemical strategies based on H1, and then their potential to be used in in vivo tumor imaging and image-guided surgery in the NIR-II region was explored. High levels of uptake were obtained for both passive and active tumor targeting probes SXH and SDH. Furthermore, high resolution imaging of blood vessels on tumors and the whole body of living mice using H1 NPs for the first time has demonstrated precise NIR-II image-guided sentinel lymph node (SLN) surgery.


 Please wait while we load your content...
Please wait while we load your content...