An efficient on-board metal-free nanocatalyst for controlled room temperature hydrogen production†
Abstract
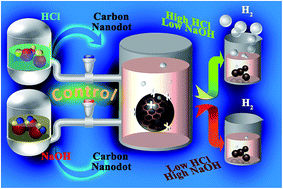
Positively charged functionalized carbon nanodots (CNDs) with a variety of different effective surface areas (ESAs) are synthesized via a cheap and time effective microwave method and applied for the generation of hydrogen via hydrolysis of sodium borohydride. To the best of our knowledge, this is the first report of metal-free controlled hydrogen generation. Our observation is that a positively charged functional group is essential for the hydrolysis for hydrogen production, but the overall activity is found to be enhanced with the ESA. A maximum value of 1066 ml g−1 min−1 as the turnover frequency is obtained which is moderate in comparison to other catalysts. However, the optimum activation energy is found to be 22.01 kJ mol−1 which is comparable to well-known high cost materials like Pt and Ru. All of the samples showed good reusability and 100% conversion even after the 10th cycle. The effect of H+ and OH− is also studied to control the on-board and on-demand hydrogen production (“on–off switching”). It is observed that H2 production decreases inversely with NaOH concentration and ceases completely when 10−1 M NaOH is added. With the addition of HCl, H2 production can be initiated again, which confirms the on/off control over production.


 Please wait while we load your content...
Please wait while we load your content...