Intra- versus intermolecular electron transfer in radical nucleophilic aromatic substitution of dihalo(hetero)arenes – a tool for estimating π-conjugation in aromatic systems†
Abstract
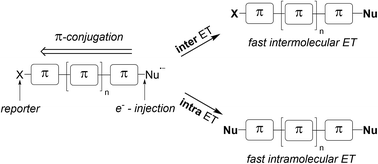
In this paper, the application of the double radical nucleophilic aromatic substitution (SRN1) in various dihalogenated, mostly diiodinated, π-conjugated systems as a tool for qualitatively estimating their π-conjugation is described. This approach uses electron delocalisation as a measure of π-conjugation. Electron injection into the π-system is achieved via reaction of an intermediate aryl radical, itself generated from a dihalogenated π-system via SET-reduction of the C–I bond and subsequent reaction with a thiolate anion. The generated arene radical anion can then further react with the second aryl-halogen moiety within the π-system via an intramolecular electron transfer process. The efficiency of this intramolecular electron transfer is related to the π-conjugation of the radical anion. If the π-conjugation within the aromatic unit is weak, the arene radical anion reacts via an intermolecular ET with the starting dihalide. The intramolecular ET process delivers a product of a double SRN1 substitution whereas the intermolecular ET pathway provides a product of a mono- SRN1 substitution. By simple product analysis of mono- versus double substitution, π-conjugation can be qualitatively evaluated. This mechanistic tool is applied to various dihalogenated π-conjugated systems and the results are discussed within the context of π-conjugation. The conjugation mode within the π-system and the length of the aromatic system are varied, and the effect of relative positioning of the two halides within small π-systems is also addressed.


 Please wait while we load your content...
Please wait while we load your content...