Shape-transformable liquid metal nanoparticles in aqueous solution†
Abstract
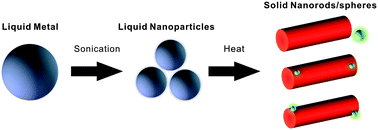
Stable suspensions of eutectic gallium indium (EGaIn) liquid metal nanoparticles form by probe-sonicating the metal in an aqueous solution. Positively-charged molecular or macromolecular surfactants in the solution, such as cetrimonium bromide or lysozyme, respectively, stabilize the suspension by interacting with the negative charges of the surface oxide that forms on the metal. The liquid metal breaks up into nanospheres via sonication, yet can transform into rods of gallium oxide monohydroxide (GaOOH) via moderate heating in solution either during or after sonication. Whereas heating typically drives phase transitions from solid to liquid (via melting), here heating drives the transformation of particles from liquid to solid via oxidation. Interestingly, indium nanoparticles form during the process of shape transformation due to the selective removal of gallium. This dealloying provides a mechanism to create indium nanoparticles at temperatures well below the melting point of indium. To demonstrate the versatility, we show that it is possible to shape transform and dealloy other alloys of gallium including ternary liquid metal alloys. Scanning transmission electron microscopy (STEM), energy-dispersive X-ray spectroscopy (EDS) mapping, and X-ray diffraction (XRD) confirm the dealloying and transformation mechanism.


 Please wait while we load your content...
Please wait while we load your content...