Regulating the topology of 2D covalent organic frameworks by the rational introduction of substituents†
Abstract
The topology of a covalent organic framework (COF) is generally believed to be dictated by the symmetries of the monomers used for the condensation reaction. In this context, the use of monomers with different symmetries is usually required to afford COFs with different topologies. Herein, we report a conceptual strategy to regulate the topology of 2D COFs by introducing alkyl substituents into the skeleton of a parent monomer. The resulting monomers, sharing the same C2 symmetry, were assembled with a D2h symmetric tetraamine to generate a dual-pore COF or single-pore COFs, depending on the sizes of the substituents, which were evidenced using PXRD studies and pore size distribution analyses. These results demonstrate that the substituent is able to exert a significant influence on the topology of COFs, which is crucial for their application.
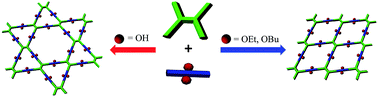


 Please wait while we load your content...
Please wait while we load your content...