Copper-catalyzed direct alkylation of heteroarenes†
Abstract
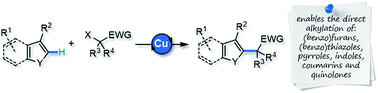
An efficient and broadly applicable process is reported for the direct alkylation of C–H bonds in heteroarenes, privileged scaffolds in many areas of science. This reaction is based on the copper-catalyzed addition of alkyl radicals generated from activated secondary and tertiary alkyl bromides to a wide range of arenes, including furans, thiophenes, pyrroles, and their benzo-fused derivatives, as well as coumarins and quinolinones.


 Please wait while we load your content...
Please wait while we load your content...