Family-level stereoselective synthesis and biological evaluation of pyrrolomorpholine spiroketal natural product antioxidants†
Abstract
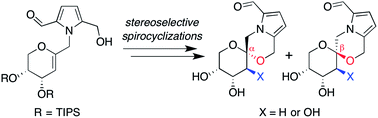
The pyranose spiroketal natural products pollenopyrroside A and shensongine A (also known as xylapyrroside A, ent-capparisine B) have been synthesized by stereoselective spirocyclizations of a common C1-functionalized glycal precursor. In conjunction with our previously reported syntheses of the corresponding furanose isomers, this provides a versatile family-level synthesis of the pyrrolomorpholine spiroketal natural products and analogues. In rat mesangial cells, hyperglycemia-induced production of reactive oxygen species, which is implicated in diabetic nephropathy, was inhibited by pollenopyrroside A and shensongine A with mid-μM IC50 values, while unnatural C2-hydroxy analogues exhibited more potent, sub-μM activity.


 Please wait while we load your content...
Please wait while we load your content...