Simultaneous engineering of an enzyme's entrance tunnel and active site: the case of monoamine oxidase MAO-N†
Abstract
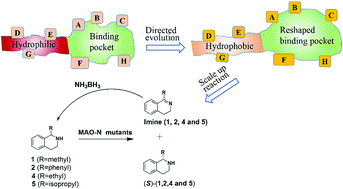
A new directed evolution approach is presented to enhance the activity of an enzyme and to manipulate stereoselectivity by focusing iterative saturation mutagenesis (ISM) simultaneously on residues lining the entrance tunnel and the binding pocket. This combined mutagenesis strategy was applied successfully to the monoamine oxidase from Aspergillus niger (MAO-N) in the reaction of sterically demanding substrates which are of interest in the synthesis of chiral pharmaceuticals based on the benzo-piperidine scaffold. Reversal of enantioselectivity of Turner-type deracemization was achieved in the synthesis of (S)-1,2,3,4-tetrahydro-1-methyl-isoquinoline, (S)-1,2,3,4-tetrahydro-1-ethylisoquinoline and (S)-1,2,3,4-tetrahydro-1-isopropylisoquinoline. Extensive molecular dynamics simulations indicate that the altered catalytic profile is due to increased hydrophobicity of the entrance tunnel acting in concert with the altered shape of the binding pocket.


 Please wait while we load your content...
Please wait while we load your content...