Template-directed synthesis of linear porphyrin oligomers: classical, Vernier and mutual Vernier†
Abstract
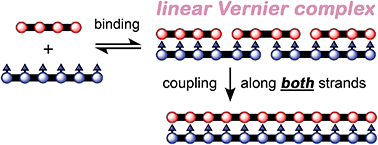
Three different types of template-directed syntheses of linear porphyrin oligomers are presented. In the classical approach the product has the same number of binding sites as the template, whereas in Vernier reactions the product has the lowest common multiple of the numbers of binding sites in the template and the building block. Mutual Vernier templating is like Vernier templating except that both strands of the Vernier complex undergo coupling simultaneously, so that it becomes impossible to say which is the ‘template’ and which is the ‘building block’. The template-directed synthesis of monodisperse linear oligomers is more difficult than that of cyclic oligomers, because the products of linear templating have reactive ends. All three types of templating are demonstrated here, and used to prepare a nickel(II) porphyrin dodecamer with 4-pyridyl substituents on all twelve porphyrin units. The stabilities and cooperativities of the double-strand complexes involved in these reactions were investigated by UV-vis-NIR titration. The four-rung ladder duplex has a stability constant of about 2 × 1018 M−1 in dichloromethane at 298 K.
- This article is part of the themed collections: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS and ISACS18: Challenges in Organic Materials and Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...