Expanding a fluorescent RNA alphabet: synthesis, photophysics and utility of isothiazole-derived purine nucleoside surrogates†
Abstract
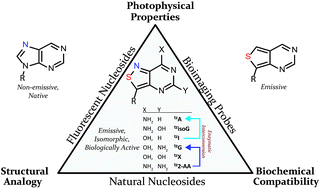
A series of emissive ribonucleoside purine mimics, all comprised of an isothiazolo[4,3-d]pyrimidine core, was prepared using a divergent pathway involving a key Thorpe–Ziegler cyclization. In addition to an adenosine and a guanosine mimic, analogues of the noncanonical xanthosine, isoguanosine, and 2-aminoadenosine were also synthesized and found to be emissive. Isothiazolo 2-aminoadenosine, an adenosine surrogate, was found to be particularly emissive and effectively deaminated by adenosine deaminase. Competitive studies with adenosine deaminase with each analogue in combination with native adenosine showed preference for the native substrate while still deaminating the isothiazolo analogues.


 Please wait while we load your content...
Please wait while we load your content...