Protein modification via alkyne hydrosilylation using a substoichiometric amount of ruthenium(ii) catalyst†‡
Abstract
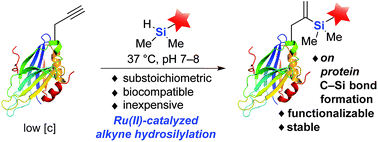
Transition metal catalysis has emerged as a powerful strategy to expand synthetic flexibility of protein modification. Herein, we report a cationic Ru(II) system that enables the first example of alkyne hydrosilylation between dimethylarylsilanes and O-propargyl-functionalized proteins using a substoichiometric amount or low-loading of Ru(II) catalyst to achieve the first C–Si bond formation on full-length substrates. The reaction proceeds under physiological conditions at a rate comparable to other widely used bioorthogonal reactions. Moreover, the resultant gem-disubstituted vinylsilane linkage can be further elaborated through thiol–ene coupling or fluoride-induced protodesilylation, demonstrating its utility in further rounds of targeted modifications.


 Please wait while we load your content...
Please wait while we load your content...