Characterization of trimethoprim resistant E. coli dihydrofolate reductase mutants by mass spectrometry and inhibition by propargyl-linked antifolates†
Abstract
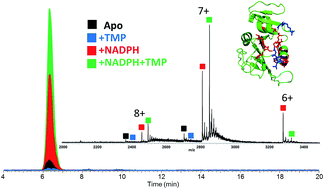
Pathogenic Escherichia coli, one of the primary causes of urinary tract infections, has shown significant resistance to the most popular antibiotic, trimethoprim (TMP), which inhibits dihydrofolate reductase (DHFR). The resistance is modulated by single point mutations of DHFR. The impact of two clinically relevant mutations, P21L and W30R, on the activity of DHFR was evaluated via measurement of Michaelis–Menten and inhibitory kinetics, and structural characterization was undertaken by native mass spectrometry with ultraviolet photodissociation (UVPD). Compared to WT-DHFR, both P21L and W30R mutants produced less stable complexes with TMP in the presence of co-factor NADPH as evidenced by the relative abundances of complexes observed in ESI mass spectra. Moreover, based on variations in the fragmentation patterns obtained by UVPD mass spectrometry of binary and ternary DHFR complexes, notable structural changes were localized to the substrate binding pocket for W30R and to the M20 loop region as well as the C-terminal portion containing the essential G–H functional loop for the P21L mutant. The results suggest that the mutations confer resistance through distinctive mechanisms. A novel propargyl-linked antifolate compound 1038 was shown to be a reasonably effective inhibitor of the P21L mutant.


 Please wait while we load your content...
Please wait while we load your content...