The development and mechanistic investigation of a palladium-catalyzed 1,3-arylfluorination of chromenes†
Abstract
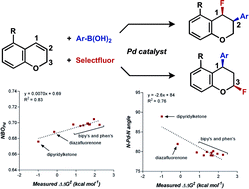
A mild palladium-catalyzed ligand-controlled regioselective 1,3-arylfluorination of 2[H]-chromenes has been developed. The products with a syn-1,3 substitution pattern were obtained with high enantiomeric excess using a PyrOx ligand, wherein the utility of these pyranyl-fluorides was further demonstrated through their participation in a diastereoselective C–C bond forming reaction. Ligand dependent divergent formation of both the 1,3- and 1,2- alkene difunctionalization products was observed. The nature of this bifurcation was investigated through experimental studies in combination with computational and statistical analysis tools. Ultimately, the site selectivity was found to rely on ligand denticity and metal electrophilicity, the electronics of the boronic acid, and the donor ability of the directing group in the substrate.
- This article is part of the themed collections: Celebrating our 2018 prize and award winners, CSC100: Celebrating Canadian Chemistry and ISACS19: Challenges in Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...