Radically promoted formation of a molecular lasso†
Abstract
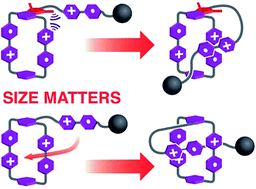
Two potential viologen-based molecular lasso precursors—both composed of a 4,4′-bipyridinium (BIPY2+) unit as part of a rope appended to a cyclobis(paraquat-p-phenylene) (CBPQT4+) loop—that have been designed to mimic the threading/unthreading motion of lasso peptides, have been synthesised and characterised. Solution and solid-state experiments reveal that, when the BIPY2+ unit in the rope and the CBPQT4+ loop are connected by a bulky linker, no lasso-like conformational transformation is observed between the different redox states on account of steric effects. In sharp contrast, when the linker size is small, the molecule can be switched between (i) a free rope-like conformation in its fully oxidised state and (ii) a self-entangled lasso-like conformation under reducing conditions employing either chemical or electrochemical stimuli: the BIPY˙+ unit in the rope resides inside the cavity of the CBPQT2(˙+) loop, forming a pseudo[1]rotaxane. The switching process is reversible and stereochemically unambiguous. This research shows how tiny structural differences can induce significantly different self-complexing properties and sheds light on designing functional artificial actuators.


 Please wait while we load your content...
Please wait while we load your content...